With no prospects for profits, big pharma neglects new infectious diseases
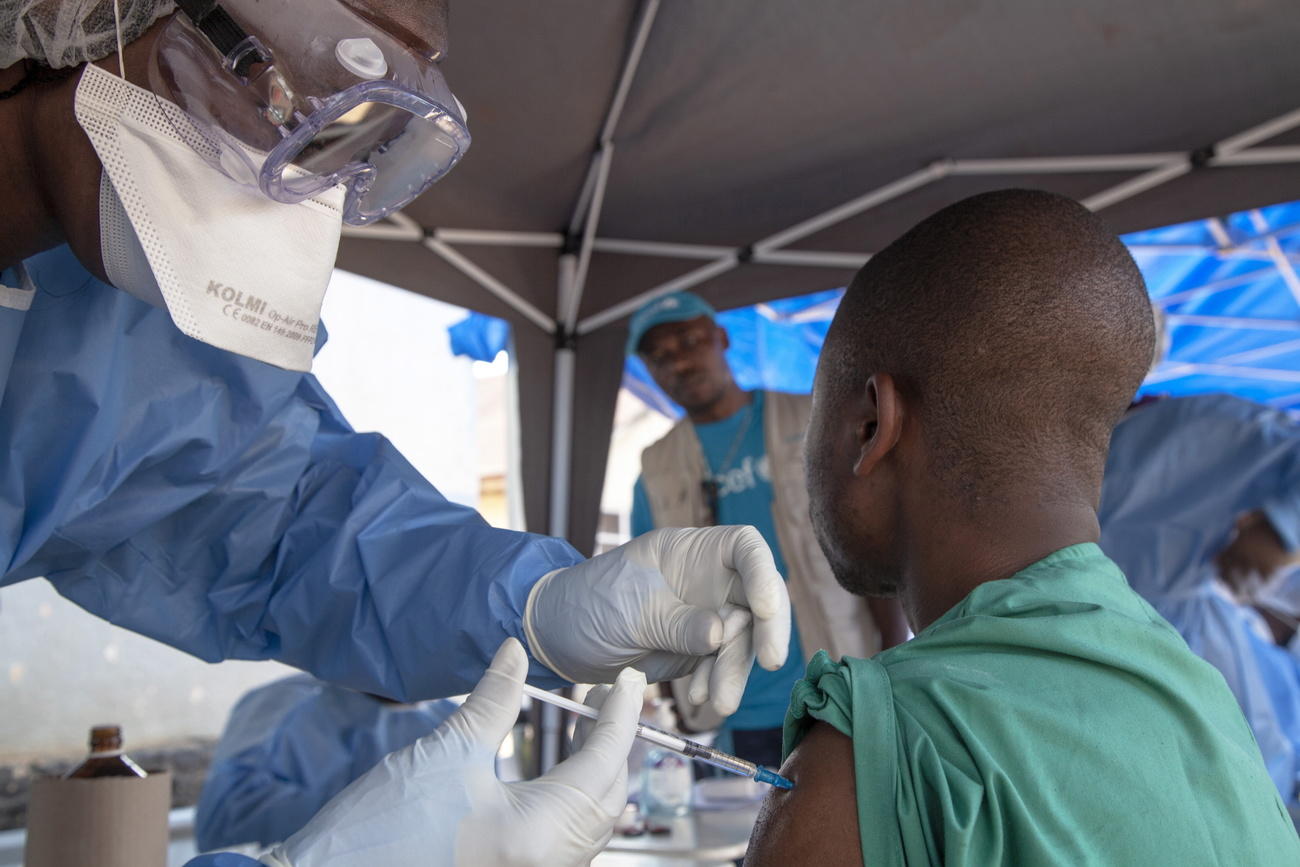
More companies, including some big Swiss drugmakers, are shifting resources away from emerging infectious diseases into more lucrative areas like cancer treatment. Their business decisions risk leaving gaping holes in the fight against epidemics such as the one caused by the novel coronavirus.
Despite Switzerland’s position as a pharmaceutical and biotech hubExternal link, companies based here have so far abstained from high-profile commitments related to the global coronavirus outbreak.
The World Health Organization (WHO) listsExternal link of Covid-19 vaccine and treatment candidates contain no Swiss company. And none of the Switzerland-based drugmakers have announced major plans to boost R&D into the virus that has so far infected around 100,000 people in some 80 countries.

More
Coronavirus: the situation in Switzerland
The wider lack of interest doesn’t come as a surprise to Bernard Pécoul, executive director of the Drugs for Neglected Diseases Initiative (DnDi), a non-profit organisation dedicated to finding treatments for diseases that are not a priority to the industry.
“A large number of big pharma companies have abandoned the field of infectious disease. It’s a big concern because we don’t think that this is the end of infectious disease as we see with the latest outbreak,” Pécoul told swissinfo.ch in Geneva, where his organisation is based.
Since its founding in 2003, DnDi has been on the front lines of efforts to attract investment in populations and diseases that are often ignored. By seeking to re-wire the market-based R&DExternal link model, the group has been able to develop eight new treatments for diseases like sleeping sickness, which threatens millions of people, largely in sub-Saharan Africa.
Their efforts are still miniscule compared to the billions pharmaceutical companies pour into cancer research and rare, deadly diseases like spinal muscular atrophy in hopes of big gene breakthroughs. At the same time, investments in emerging infectious diseases – those outside HIV, malaria and tuberculosis – have dwindled.
+ Swiss foundation launches ‘emergency call’ for coronavirus research
The latest Access to Medicines IndexExternal link found that nearly half of R&D projects by the 20 biggest pharmaceutical companies target cancer while there were no projects on coronaviruses (MERS-Cov and SARS-Cov) at the time the report was published.
Novartis sold its vaccine divisionExternal link to British pharma company GSK in 2014 after operating at a loss for years. The company no longer has critical mass of expertise in virology, and no laboratories are working on antivirals or diagnostics. Consolidation in the vaccine business has left four big players controlling some 80% of the nearly $45 billion (CHF43 billion) market.
“Companies concentrate on the market that is attractive in terms of profit. Oncology is a market that has been very profitable. Now even orphan diseases [rare disease classification by the US government] are marketed as profitable because they could offer a price that is very high.” says Pécoul.
Investment in coronaviruses
Policy Cures ResearchExternal link, a health thinktank in Australia, has been tracking global R&D investment in emerging infectious diseases.
Preliminary findings of their report that will be released later this year, suggests that overall coronavirus R&D funding (focusing on MERS, but including SARS R&D and research targeting multiple coronaviruses) was US $27 million in 2016, grew to $50 million in 2017 and fell significantly, to around $36 million in 2018 – far below the levels of funding received by Ebola and Zika.
Paul Barnsley a Senior Analyst at Policy Cures, told swissinfo.ch that “there has been very little reported private sector funding for coronavirus R&D over this period.” But qualifies that the nature of investment highly depends on whether there is an outbreaks, and the ability to conduct clinical trials.
“The small share of private funding for coronavirus R&D likely reflects, in part, the absence of opportunities for clinical testing over the period covered by our data”, Barnsley explains.
Diverging priorities
Ellen ‘t Hoen, director of Medicines Law and PolicyExternal link, argues that “pharmaceutical companies don’t necessarily set priorities according to what global health priorities are.” The trained lawyer who worked for Doctors without Borders and the WHO says that shareholders are used to big rewards and their priorities don’t often converge with public health priorities.
But some companies argue this explanation is too black and white, pointing out that cancerExternal link is still the second-leading cause of death globally and chronic diseases like diabetesExternal link are on the rise.
In an interview on the sidelines of the World Economic Forum in January, Harald Nusser, who leads Novartis Social Business, told swissinfo.ch that the company must consider where its pipeline and experience can make the most meaningful contribution. It is heavily investedExternal link in treatments for tropical diseases such as malaria, leprosy and leishmaniasis.
“These may at times not be the biggest public health threats or need at this very moment, but people are still dying of them,” says Nusser.
The trouble with epidemics
Epidemics pose a unique challenge to pharma executives. There is a lot of activity when an outbreak happens but when it tapers off, so does the investment. This means that “promising medical technologies may fall by the wayside because there is no one ready to pick up the bill,” says ‘t Hoen.
This was echoed by Novartis CEO Vasant Narasimhan in an interview on CNBCExternal link earlier this year, who said “there is a lot of interest and activity but then a lot isn’t happening and people lose interest and the investment flows out. So, the question is how do you keep the investment in place in the troughs of interest in pandemics, and these kind of outbreaks?”
GSK’s experience in Ebola is one often-cited cautionary taleExternal link. After investing for years in three vaccines, progress stalled in the final stage of clinical trials toward the end of the 2014-16 epidemic, due to a dwindling number of Ebola cases. With no real prospect of a financial return, the company eventually gave up and handed the candidates over to a non-profit institute in the US last year. This was despite an Ebola outbreak at the time in the Democratic Republic of the Congo.
+ A Swiss lab made the first synthetic clone of Covid-19
Companies suffered a similar fate during SARS, said Thomas Cueni, who heads the International Federation of Pharmaceutical Manufacturers, on Swiss public television RTS. External link “Some 17 years ago, there were companies which started to develop vaccines. But when it was time for clinical tests, there were no more patients, because the virus was gone.”
These experiences have likely made companiesExternal link more cautious about diving head-first into the search for novel coronavirus vaccinations or Covid-19 therapies. Many companiesExternal link have been donating supplies and offering advice to local and global health authorities. Novartis, Johnson & Johnson and Sanofi have indicated that they are reviewing existing products to see if they can be repurposed for the coronavirus.
Roche’s arthritis drug Actemra has been added to the diagnosis and treatment plan for Covid-19 issued by the China National Health Commission on March 3 as a possible therapy for seriously ill patients. The company is also working with a German company that uses Roche’s LightCycler® 480 System to speed up the diagnosis of coronavirus infections.
A company spokesperson told swissinfo.ch that it is delivering as many tests as possible within the limits of supply.
While these efforts are significant, vaccine development would involve much larger financial commitments on very short notice with very little prospects of a financial return. That urgency also brings risks, including legal liability for the companies.
Many investors are placing betsExternal link on smaller companies that are more willing to take on the risks. When little-known company Vaxart announced it was searching for a possible Covid-19 vaccine, its stock jumped 106.1%. Shares also rose for other biotechs such as Novovax and Inovio after they announced plans for testing and trials.
The WHO predicts a coronavirus vaccine would take 18 months to make, which is less than the typical vaccine development process.
Fixing a broken model
Looking back on a 35-year-long career in global health, ‘t Hoen fears that we still haven’t learned the lessons from the past. “The coronavirus seems poised to join a lengthy list of health problems the industry turns its back on unless additional incentives are made available,” she wrote in a recent commentary in BarronsExternal link.
Ultimately some kind of public-private partnership is going to save the day, says ‘t Hoen. She just hopes that affordability is addressed up front and manufacturers don’t receive exclusive rights. This was already a source of tension in the Coalition for Epidemic Preparedness Innovations,External link which is seen as promising effort to drive investment into vaccine R&D for epidemics.
The group has struggled to get pharmaceutical firmsExternal link to agree to be partners without insisting on substantial profits or proprietary rights to research that CEPI, funded largely by governments and charities like the Bill & Melinda Gates Foundation, helped finance and produce.
Pécoul would hate to see a solution whereby companies simply donate drugs and offer up some money. “We need some commitment that is much stronger than charity.”

More
Swiss pharma giants swallow up start-ups in push for next big gene therapy

In compliance with the JTI standards
More: SWI swissinfo.ch certified by the Journalism Trust Initiative


You can find an overview of ongoing debates with our journalists here. Please join us!
If you want to start a conversation about a topic raised in this article or want to report factual errors, email us at english@swissinfo.ch.